35+ Calculating Molar Solubility From Ksp
You will also calculate. The first thing to do is identify the values of n and m by writing the dissociation equilibrium for magnesium hydroxide MgOH2s Mg2 aq 2OH aq As you can see.
17 2 Molar Solubility And Ksp Chemistry Libretexts
Web Is Ksp the same as molar solubility.

. Its also important to note for this lead two fluoride problem if the pH is decreased at a constant temperature the Ksp value for PbF two remains constant. Calculating Solubility from Ksp Read Chemistry CK-12 Foundation Calculating Solubility from Ksp Demonstrates how solubility constants can be derived from experimentally. It explains how to.
Tabulate the initial conditions including the given amount of common ion. Calculating the Ksp from Molar Solubility 3 There is a 11 molar ratio between the AgBr that dissolves and Ag that is in solution. So the molar solubility does.
Web Is Ksp the same as molar solubility. All Modalities Calculating Ksp from Solubility. In like manner there is a 11 molar.
Write the chemical equation for the salts solubility reaction. Tabulate the initial conditions including the given amount of common ion. Let S the solubility saturation concentration of the Ca 2 ion in moles per liter.
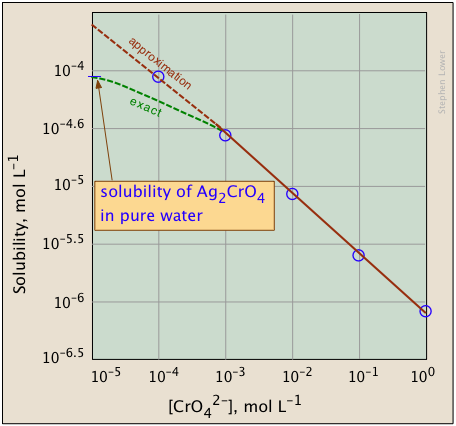
Solution The relavant equilibrium is A g 2 C r O 4 s 2 A g a q C r O 4 a q 2 so the associated equilibrium constant is K s p A g 2 C r O 4 2 Important. Calculating Ksp from Solubility Demonstrates calculations used to relate solubility constants to solute concentration. Determine the K sp of silver bromide given that its molar solubility is 571 x 10 7 moles per liter.
The Ksp expression is. Tabulate the initial conditions. Ksp Ag2CO3 81 x 10-12 Round the answer to two significant digits.
AgBr s Ag aq. Science Chemistry Calculate the molar solubility of silver carbonate. Then 2S the solubility saturation concentration of the F - ion.
Solubility Product Constant Objective. In this experiment you will determine the solubility product constant of CaIO32 in a saturated solution of calcium iodate. 305K views 1 year ago New AP General Chemistry Video Playlist This chemistry video tutorial provides a basic introduction into Ksp - the solublity product constant.
K s p represents how much of the solute will dissolve in solution and the more soluble a substance is the higher the chemistry K s p value. Write the equation for the compounds solubility reaction. Substitute S into the.
To calculate the solubility. Steps for Using Ksp to Calculate the Solubility of a Compound Step 1. 1 When AgBr dissolves it dissociates like this.
11 x 10-4 M O 13 x 10-4 M O 15 x 10-¹4 M 17.

Solubility Product Constant Ksp Overview Formula How To Calculate Ksp Video Lesson Transcript Study Com

Determine Ksp Given Solubility Youtube

Calculating Solubility From Kₛₚ Worked Example Video Khan Academy

How To Calculate Molar Solubility From Ksp In A Solution Homework Study Com

Calculate Solubility Product Constant Ksp From Molar Solubility 001 Youtube
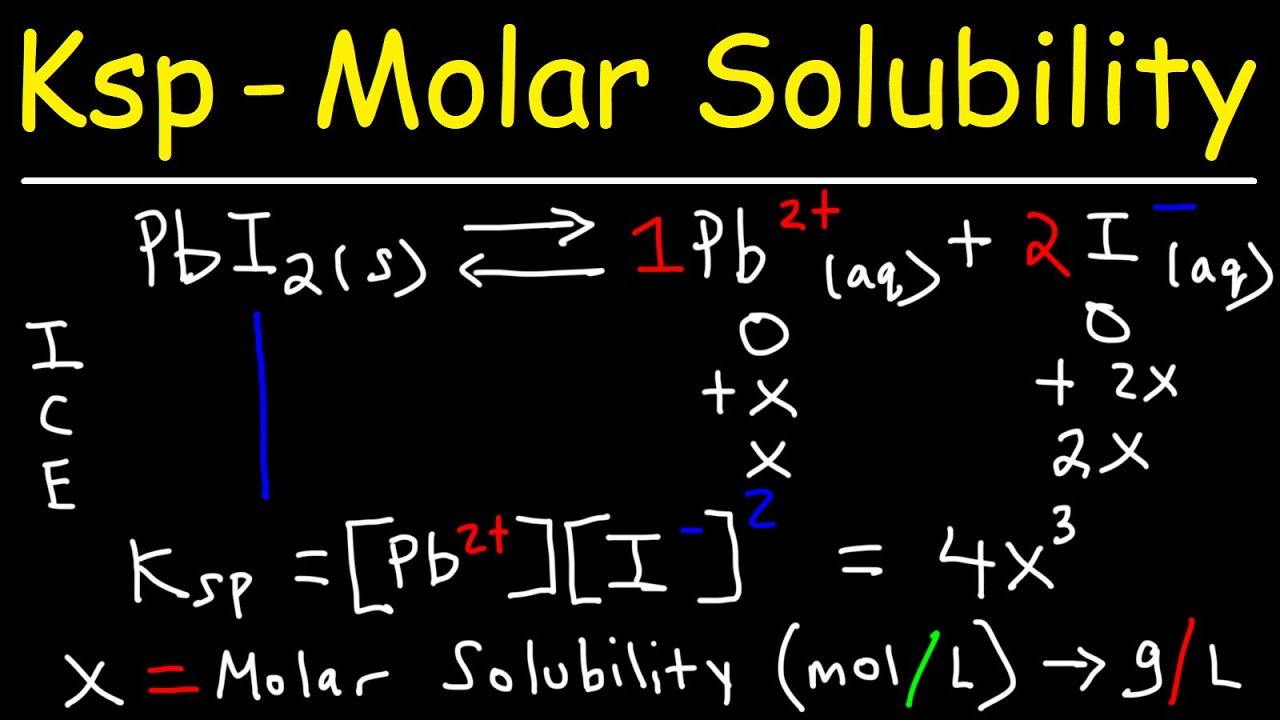
Ksp Molar Solubility Ice Tables Common Ion Effect Youtube

Calculate Ksp From Molar Solubility Problem 86 Youtube

How Will You Calculate Solubility Product From Molar Solubility
Solubility Product

79 School Ideas Text Analysis Diagramming Sentences School

Concentration Solubility Teaching Resources Tpt

Solubility Concentration Teaching Resources Tpt

Using The Solubility Of A Compound To Calculate Ksp Chemistry Study Com

Calculating Molar Solubility From Ksp

Calculating Ksp From Molar Solubility Example Calculation Youtube

1st Puc Kseeb Solutions

Chem 201 Lab Manual 2021 Spring By Fgarces Issuu